About us
Neowise Biotechnology is an innovative biotechnology company developing TCR-based cell therapy drugs for the treatment of solid tumors.
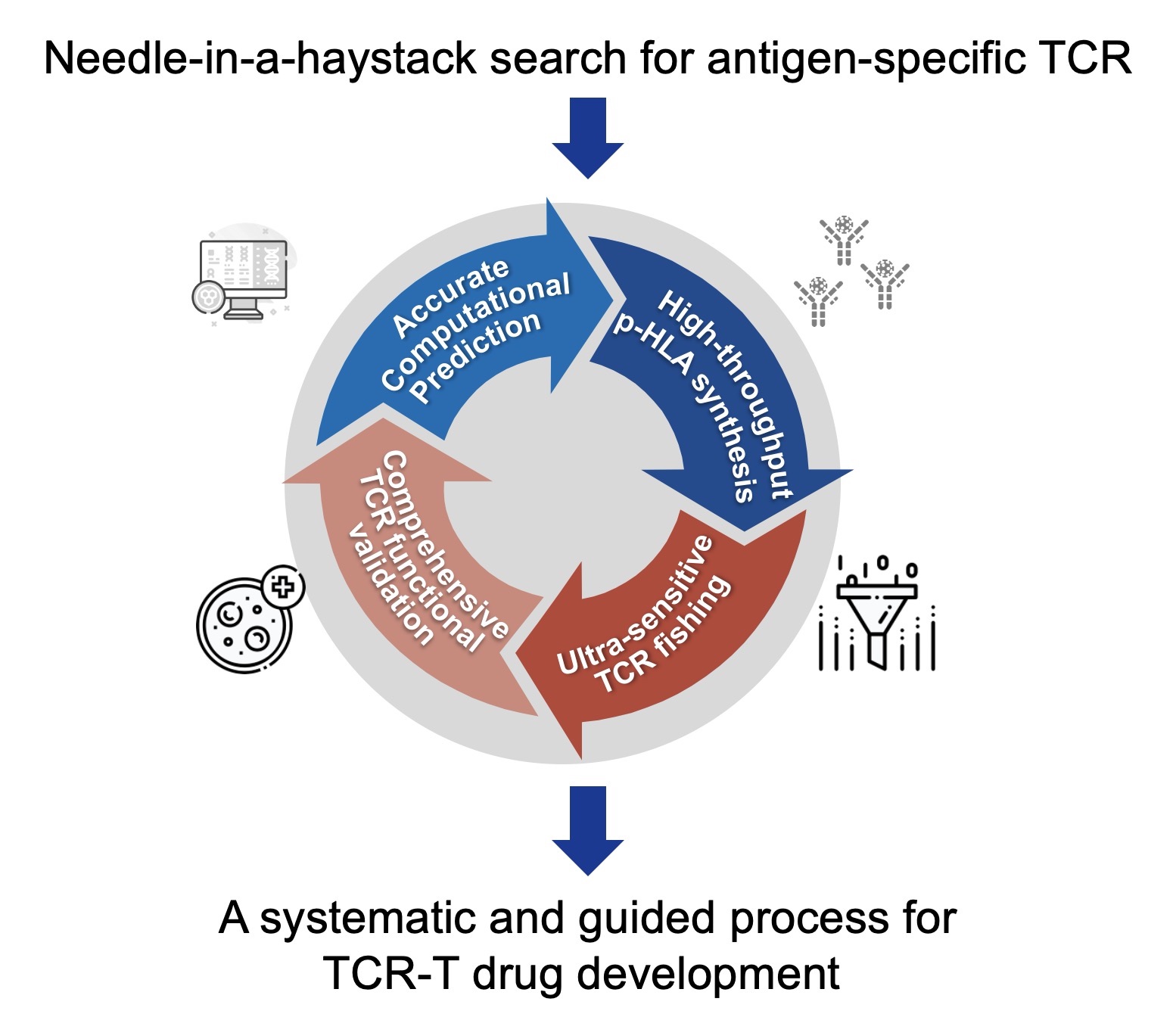
Founded in 2020, Neowise has built a high-throughput and ultra-sensitive platform for discovering natural TCRs that specifically recognize tumor antigens. By iteratively integrating computational analyses with experimental validation, the platform efficiently screens for high-affinity tumor antigen-specific natural TCRs. Using this platform, Neowise has established a a globally leading experimentally validated tumor antigen-natural TCR pairing library, CAST®. This signifcantly expanded the landscape of therapeutic targets and treatment options for patients with solid tumors. Several of the company's pipelines have advanced into clinical trials, where encouraging efficacy and favorable safety profiles have been observed across multiple solid tumor indications.
History
2024
Multiple patents granted
Key patents for PRAME and KRAS mutation targeting TCRs granted.
Entered clinical stage
Several pipelines advanced into investigator-initiated clinical trials.
2023
Completed Series A+ Funding
Raised Series A+ financing of nearly 200 million RMB.
TCR discovery and validation continues
Over 7,000 antigen-TCR pairs in the CAST library
2022
TCR discovery runs smoothly
Accumulated over 1,000 antigen-TCR pairs in the CAST library.
Functional validation ongoing
Efficiently validating the functions of the discovered TCRs, and selecting the best TCRs to go into the clinic.
Over 3,500 m2 GMP-ready factory
Built a GMP-ready facility of over 3,500 m2 to support process development and CMC.
2021
Research center ready
Early-stage R&D works progressing steadily.
TCR discovery platform established
Obtained about 100 TCR-antigen pairs.
Completed Series A Funding
Raised Series A financing of nearly 100 million RMB.
2020
Company founded
Neowise was founded in Suzhou, China in Sep 2020.
Completed Angel Funding
Raised Angel round financing of tens of millions RMB.















